- SEGMENTS
- AUTOMATION SOLUTIONS
- CAPABILITIES
- SERVICES
- RESOURCES
- About Us
- Brands & Affiliates
- Quality
- Environment
- Locations
- Careers
- Contact
- SEGMENTS
- AUTOMATION SOLUTIONS
- CAPABILITIES
- SERVICES
- RESOURCES

Segments
From contact lenses to mail-order pharmacies, ATS Life Sciences Systems has a long history of providing automated manufacturing solutions for the assembly and handling of a broad range of products.

Automation Solutions
We’ll custom design or help you find the right machines for your Assembly, Material Handling, Conveyance, Manufacturing, Vision Testing, Software (IIoT) needs

SERVICES
Our ability to assist you with your project begins with understanding your product and processes. Whatever the stage of your product’s life cycle—product design, product iteration, clinical trials, or full commercial production—ATS Life Sciences Systems can complement your staff with CGMP-experienced consultants, engineers, and skilled trades and service people

Resources
A deeper dive into information and details about LSS solutions, from the experts who work on them every day.
Sign up for Life Sciences News and Updates

Precise irradiation without harmful chemicals
E-BEAM STERILIZATION
Electron beam sterilization is an ISO-certified process delivering precise doses, preserving the quality of medical devices and medtech without heat or extended radiation exposure
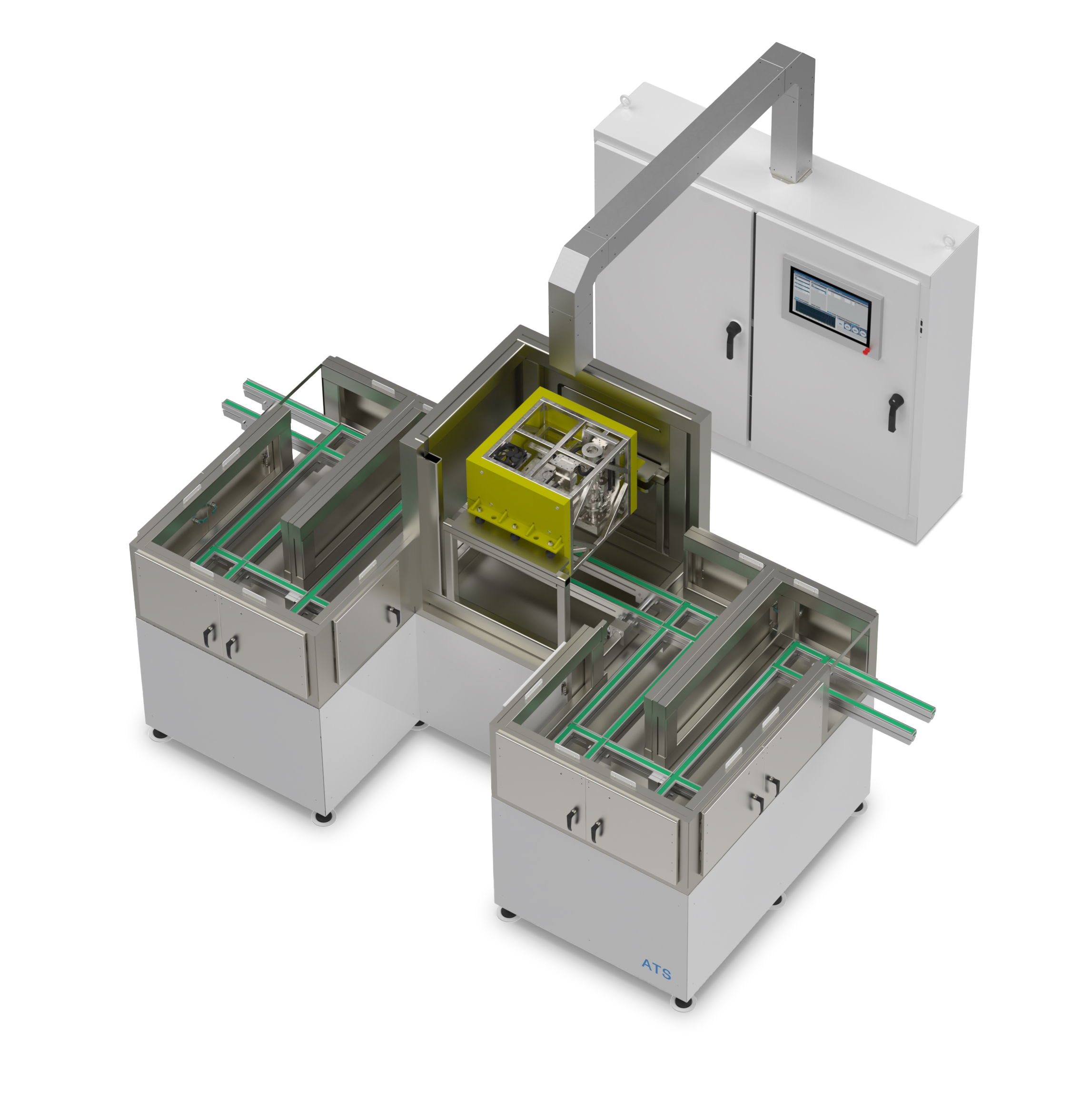
Small Batch Sterilization
Scalable integrated solutions allow medical devices and medtech to be sterilized as individual products
- Greater protection for operators and the environment without radioactive or carcinogenic materials
- Right-size the solution for integrated in-line or self-contained treatment
- Conventional power sources offer consistency against changing legislation and permitting
- Products can be released immediately following treatment
Why Consider E-beam Sterilization in Your Automation Process
Smaller units are capable of treating individual products rather than full boxes, allowing for smaller power sources, and conveyors that are easier to shield
Transitioning away from older technology like gamma, xray, or EO is simplified; E-beam addresses concerns including sustainability, stable access to materials, permitting, operator safety, energy use, and environmental factors.
Comparing Sterilization Modalities*
Comparing sterilization modalities is essential to help make informed decisions about which sterilization method is most appropriate for specific products, taking into account factors such as effectiveness, material compatibility, processing time, cost, and environmental impact.
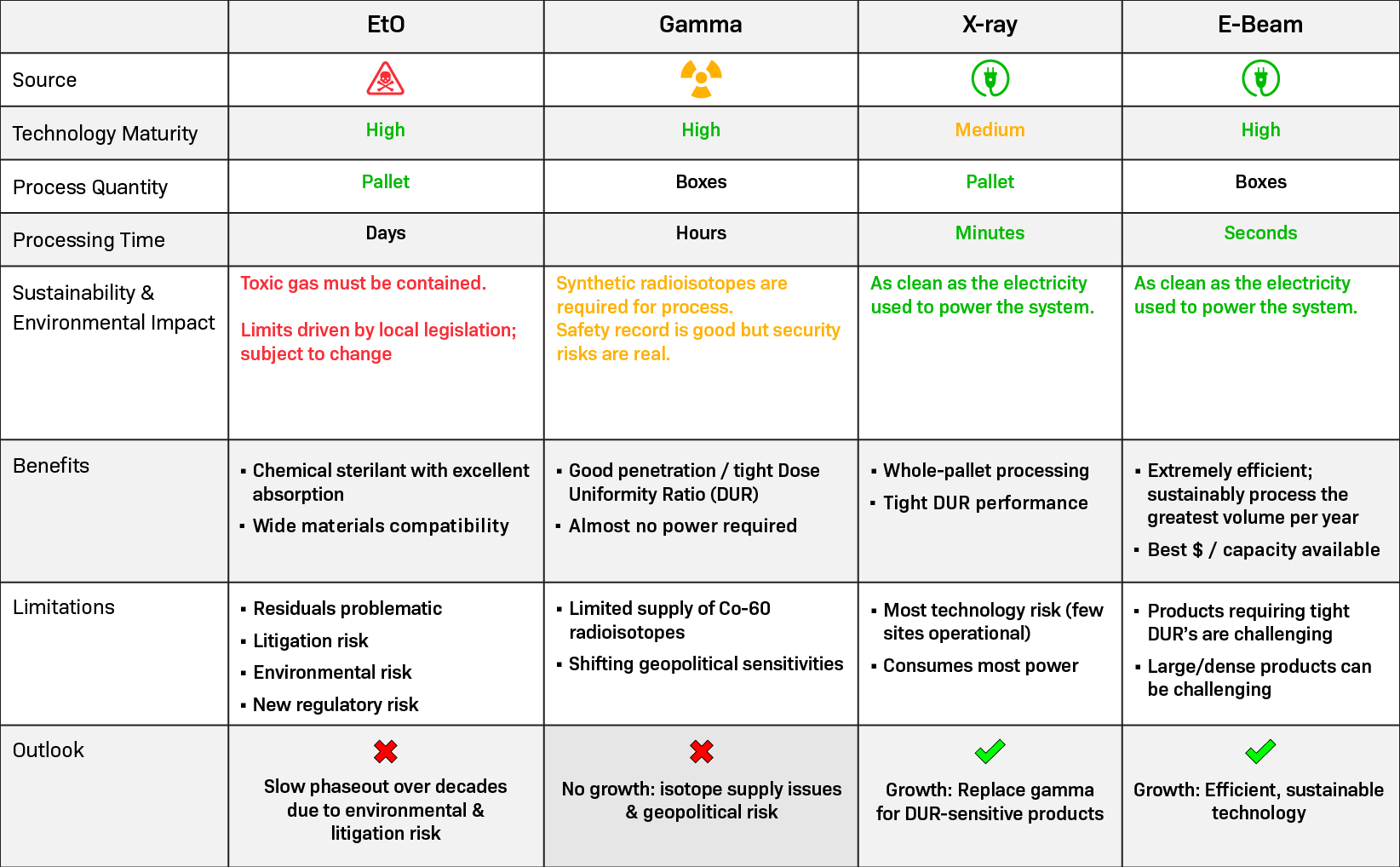
*Reproduced with permission of NextBeam
A Look Back at ATS Life Sciences Systems’ First E-beam Sterilization Symposium
See the experts’ presentations underscoring three key benefits of E-beam sterilization:
- No radioactive or carcinogenic materials – Unlike gamma irradiation or ethylene oxide sterilization, E-beam technology does not rely on hazardous substances, making it a safer and more sustainable option.
- Conventional power sources protect against changing legislation – As global regulations continue to evolve, companies that adopt E-beam sterilization can future-proof their operations and maintain regulatory compliance.
- Immediate product release – With traditional sterilization methods, products often require extended quarantine periods before distribution. E-beam sterilization eliminates this bottleneck, accelerating time-to-market.
WATCH NOW
WATCH NOW
Why MedTech Industry Leaders Are Choosing E-beam*
 Precise and Efficient
Precise and Efficient
E-beam provides precise irradiation in seconds, leaving no harmful chemical residuals. Right sizing the E-beam solution allows for treating individual products, rather than boxes, and smaller power sources and conveyors that are easier to shield.
 Enhanced Material Compatibility
Enhanced Material Compatibility
E-beam’s quick dose delivery minimizes material degradation, making it ideal for sterilizing radiation-compatible medical devices without prolonged oxidative exposure.
 Sustainable & Safe
Sustainable & Safe
E-beam sterilization uses conventional power, offering a safer and greener alternative to Gamma and Ethylene Oxide (EO), without carcinogenic or radioactive materials.
 Reliable Results
Reliable Results
This ISO-certified process delivers precise, batch-scale sterilization, preserving the quality of medical devices without heat or extended radiation exposure.
*Content reproduced with permission of NextBeam
To learn more about this technology, get to know our partners

Where Advanced Accelerator Science and real-world Solutions Converge
RadiaBeam is unique in the particle accelerator field due to the synergy between the research and commercial sides of our business. Each enhances the other. We apply multi-disciplinary expertise at the frontiers of science to produce solutions that work. We transform ideas into physical reality.
STERILIZE, TREAT, OR TEST WITHOUT HIGH-RISK ISOTOPES OR CHEMICALS**
ATS Life Sciences Systems is working with industry experts NextBeam and RadiaBeam to provide sustainable and reliable solutions to manufacturers of medical devices and medtech. Scalable and configurable solutions allow for end-of-line treatment or integrated assembly and sterilization on the same chassis.
These irradiators are based on innovative particle accelerator technology, an electronic source, making them an effective replacement for radioisotopes and toxic gases (Ethylene Oxide), and producing more adequate energy than X-ray tubes for effective penetration. Electron beam irradiators deliver high energy for a wide range of applications safely, uniformly, and efficiently.
RIGHT-SIZE TO SELF-CONTAINED, COMPACT IRRADIATORS IN VARIOUS CONFIGURATIONS
Self-contained irradiators can be configured to fit various applications including:
- surface irradiation
- in-line irradiation of medical devices/medtech
- energy variability
- types of output (electrons or X-rays)
- dosing schemes
- types of beam spreading, and
- beam orientation.
MEDICAL DEVICE STERILIZATION: REPLACE HIGH-RISK EO WITH HIGH-ENERGY ELECTRONS
Replacement of Ethelyne Oxide (EO) has become a priority for medical device sterilization facilities where unsafe levels of EO released into the environment can impact worker and public safety. EO off gassing from ‘hot pallets’ post-aeration can continue at every point in the supply chain: transportation, distribution centers, and destination warehouses. An E-beam alternative is a clean and safe irradiation solution.
A NEW LEVEL OF SAFETY AND PROCESSING EFFICIENCY
Linac-based electron beam sterilization solves the growing health concerns around use of Ethylene Oxide (EO) and rising costs and limited supply of Co-60 gamma irradiation. Unlike EO, products can be released immediately and without the decreasing capacity issues of gamma irradiation. Safe, high-performance E-beam solutions are available with the flexibility to offer components or complete irradiator systems for medical device sterilization.
**Content courtesy of RadiaBeam
Resources

Innovation in Action: A Look Back at ATS LSS First E-Beam Sterilization Symposium
ATS Life Sciences Systems hosted its first-ever E-Beam Sterilization Symposium to explore the future of sterilization technology. The event showcased the advantages of E-beam sterilization, such as safety, regulatory adaptability, and faster product release, setting a new standard for industry collaboration.
Ready to make the transition to more sustainable sterilization?
Learn how this technology can safeguard your high-volume single-use devices, and highly-engineered devices. Future-proof your production by understanding E-beam today.
TALK TO AN EXPERT
Briefly tell us about your automation needs and we’ll get back to you.
GET IN TOUCH
Briefly tell us about your automation needs and we’ll get back to you.
👋 Looking for something? I'm Gears and am happy to help.





 Contact Us
Contact Us  Subscribe
Subscribe  LinkedIn
LinkedIn  Youtube
Youtube